Coulomb measured the force between two point
charges and found that it varied
inversely as the square of the distance between the charges and was directly
proportional to the product of the magnitude of the two charges and acted along
the line joining the two charges.
Thus, if two point charges q1, q2 are separated by a distance r in vacuum, the magnitude of the force (F) between them is given by
The choice of k determines the size of the unit of charge. In SI units, the
value of k is about 9 × 109. Putting this value of k in above Equation, we see
that for q1 = q2 = 1 C, r = 1 m F = 9 × 109 N
That is, 1 C is the charge that when placed at a
distance of 1 m from another charge of the same magnitude in vacuum experiences
an electrical force of repulsion of magnitude 9 × 109 N.
The constant k in is usually put as

for later convenience, so that Coulomb’s law is written as
The value of Permittivity of free space in SI units is equal to 8.854 × 10–12 C2 N–1m–2



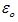
Very helpfull.. nd concept clearing .....
ReplyDeletegood
ReplyDelete